Objectives:
- Describe light in terms of wavelength and energy. Understand characteristics of the Bohr and Quantum Mechanical model of the atom.
- Use the periodic table, electron configurations, and correctly apply the rules for electron filling in atoms, to identify elements.
- Understand how Mendeleev and Moseley organized the periodic table, and know how the current periodic table is arranged Calculate the average atomic mass of an element.
- Understand the characteristics and location of metals, nonmetals, and metalloids on the periodic table.
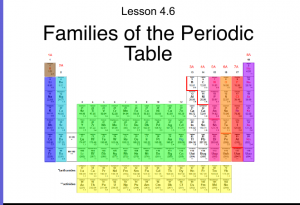
- Know the characteristics, names, and location of the families on the periodic table.
- Understand the periodic trends atomic radius, ionization energy and electronegativity and predict the reactivity of an element.