Extra Practice for Thermochemistry
Unit 11 Review
Learning Objectives
- Thermochemistry
- Distinguish exothermic and endothermic processes based on movement of energy
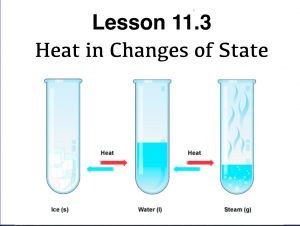
- Describe the relationship between temperature and states of matter as outlined by warming (cooling) curves
- Calculate heat changes for changes in temperature and states of matter using enthalpy equations
- Reaction Rates
- Use collision theory to describe how chemical reactions are based on the motion and arrangement of reactant particles
- Identify factors that affect reaction rates: temperature, concentration, surface area and catalysts
- Interpret an energy diagram for a reaction
- Chemical Equilibrium
- Describe a system in equilibrium using equilibrium chemical equations
- Write an equilibrium expression for a system in equilibrium (Keq)
- Using Le Châtelier’s principle, describe the shift that results from changes in concentration, temperature, or pressure of a system in equilibrium