Unit 5 Packet
Unit 5 Review
Learning Objectives
- Ionic compounds are formed through the transfer of electrons
- Explain how elements form ions through the loss or gain of electrons
- Determine the charge of the ion of elements based on the relative position on the periodic table and their electron configurations
- Predict formulas for ionic compounds
- Determine names for ionic compounds given chemical formulas
- Molecules are composed of atoms that share electrons by overlapping valence shells
- Explain how electrons are shared in covalent bonding
- Determine names for covalent compounds given molecular formulas
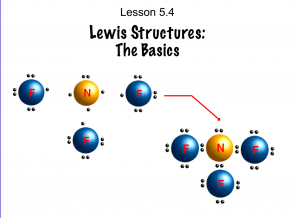
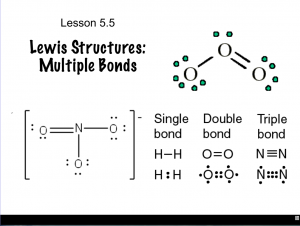
- Determine Lewis structures given molecular formulas
- Differentiate between types of covalent bonds based on the number of shared electrons